
Theory and chemistry – an overview
The chemistry of cement and concrete include many different chemical reactions to achieve the specific properties desired in concrete. Different raw materials and additives are also used that can influence the chemical reactions.
Portland cement is made mainly of four different components; calcium, silicon, aluminum, and iron. These are supplied in cement production through various raw materials such as limestone, clay, marl, silica sand, shale, etc. (the raw mix). A main raw material in cement is limestone (CaCO3). In the cement kiln, the raw materials are heated up and CO2 is driven off in the calcination reaction mainly from CaCO3 according to reaction (1). In the calcination reaction, CO2 is released to the atmosphere in the exhaust gases from the cement kiln. CaO forms an integral part of the cement mainly as various calcium silicates, calcium aluminates, and calcium ferrites. Only a small part occurs as free CaO.
CaCO3 + heat → CaO + CO2 (1)
In the manufacture of concrete, water is added to cement to form the cement paste (hydration process). The added water reacts with different substances in the cement such as tricalcium silicates and dicalcium silicates to form hydration products such as calcium silicate hydrate (C-S-H) and also calcium hydroxide (Ca(OH)2). The carbonation reaction is often written, for simplicity, with Ca(OH)2 but CO2 also will react with other components, such as the C-S-H gel.
Thus, both C-S-H gel and Ca(OH)2 form a part of the cured concrete. CO2 mainly in the atmosphere, in contact with concrete, will primarily react with Ca(OH)2 in the concrete according to the principle reaction (2) but will also react with the C-S-H gel. These reactions represent the uptake of CO2 in concrete, which is called carbonation.
Ca(OH)2 + CO2 → CaCO3 + H2O (2)
CO2 is a natural part of the atmosphere. However, the concentration of CO2 in the atmosphere is increasing due to an extensive global use of fossil fuels. The concentration of CO2 in the atmosphere has increased from about 280 ppm in preindustrial time to about 400 ppm today. The rate of concentration increase in the atmosphere is today about 1-2 ppm/year. An increased concentration of CO2 in the atmosphere can, to some extent, also increase the rate of carbonation even if the contribution is small on a yearly basis.
The carbonation reaction takes place in several steps. The actual uptake reaction is the reaction between the calcium and carbonate ions (3). This reaction takes place in water phase in the pore solution in the concrete. Water and moisture are thus important parts of carbonation.
Ca2+ + CO32- → CaCO3 (3)
In the alkaline pore water solution in concrete, portlandite (calcium hydroxide or Ca(OH)2) can be dissolved according to reaction (4) forming calcium and hydroxide ions.
Ca(OH)2 → Ca²+ + 2OH- (4)
CO2 is also dissolved in the alkaline pore water solution according to reaction (5) forming carbonic acid (H2CO3).
CO2 + H2O → H2CO3 (5)
The protolysis of H2CO3 in alkaline solution proceeds in two steps, (6) and (7) forming bicarbonate (HCO3-) and carbonate (CO32-) ions.
H2CO3 + OH- ↔ HCO-3 + H2O (6)
HCO-3 + OH- ↔ CO32- + H2O (at high pH, in uncarbonated concrete) (7)
In this way, Ca2+ and CO32- are formed and can react and precipitate as limestone (CaCO3) in the concrete.
The carbonation rate
An important aspect is the carbonation rate. How fast can the carbonation proceed, and which are the rate determining processes? When estimating the CO2 uptake in the carbonated concrete, one must also consider the degree of carbonation.
The carbonation rate depends on several factors such as the chemical reaction rate, mass transport of CO2, humidity, temperature, porosity, CO2 concentration in ambient air etc. The rate determining step could be crucial to the overall uptake rate. For practical reasons, the carbonation rate is often determined by measuring the depth of carbonation as a function of time. The depth is proportional to the square root of time. The carbonation rate can then be expressed as a constant in mm/√year.
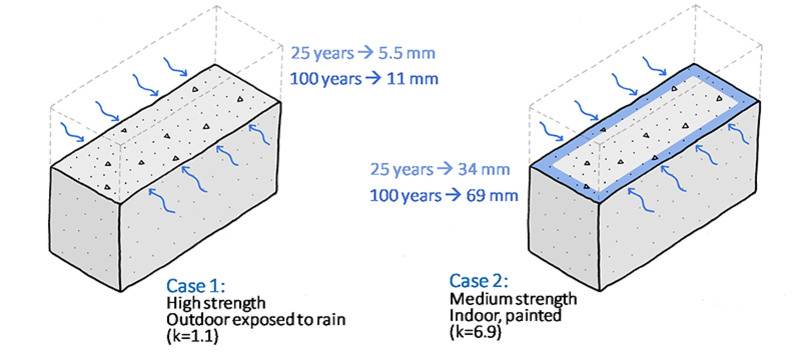
The carbonation rate, expressed here as the depth of carbonation, can be very different depending on several concrete and surface exposure factors. The k factor is given in mm/√year.
Degree of carbonation
The degree of carbonation, DOC, is defined as the amount of CO2 taken up in relation to the maximum CO2 uptake in the carbonated volume of the concrete. The maximum uptake of CO2 can often be equated with the CO2 emission from calcination that is driven off from the material. Traditionally, the degree of carbonation has been defined only within the area which has been considered carbonated and this area has been defined as the area exhibiting color change with a phenolphthalein test. (This test reveals where the depth within a cross section of concrete at which the pH has been reduced by carbonation to below about 9.) However, when calculating CO2 uptake in concrete or other cement-containing products, there may be a need for a more general definition of degree of carbonation that includes all CO2 reacted within the concrete (including that beyond the pH change region) in relation to all concrete.
In general, one can say that the chemical reactions presented above are relatively fast and cannot be considered as the rate determining step. However, there are several other factors that can slow down the carbonation rate. A common aspect for those factors is the access to, and transport of, molecules through the concrete. As carbonation proceeds, more and more CaCO3 is precipitated in the concrete which can reduce the permeability of the concrete. This will reduce the access of CO2 to the interior of the concrete, slow down the dissolution of Ca(OH)2 and thus decrease the carbonation rate.
Water is required
Water is required for carbonation to take place. Concrete is a porous material that allows both CO2 in air and water to penetrate into the concrete. The CO2 gas in the pores will dissolve in the water in the pores and carbonation can start. However, if the pores are completely filled with water, the HCO3- or CO32- ions have to diffuse in the water phase into the concrete. This is a much slower process and will thus slow down the carbonation rate. Obviously, there is an optimal moisture content in concrete for a maximum carbonation rate. The optimal moisture content in concrete for carbonation has been estimated to be about 60-80 % relative humidity in the concrete.
Carbonation rate will slow down
Mainly due to the formation of CaCO3 in the concrete, the carbonation rate will slow down with time. Empirical experiments have shown that the carbonation rate is proportional to the square-root of time(t). Other factors that will influence the carbonation rate are porosity of the concrete, w/c ratio, cracks in the concrete, cement type and additives, and surface treatment of the concrete products.
At the end-of-life of concrete products, they are demolished and often crushed for recycling purposes. When the concrete is crushed, new surfaces are created and most of those surfaces were previously in the interior of the concrete, with limited exposure to CO2. This can dramatically increase the carbonation rate if access to CO2 in air can be maintained. These smaller concrete pieces will also increase the total carbonation in an entire concrete volume. To estimate the uptake of CO2 in concrete, it is thus important to include both the service life of the concrete products and the secondary use of the concrete after the end-of-life phase.

